For over a decade, Drs. Edward Damiano (Associate Professor, Biomedical Engineering) and Firas El-Khatib (Senior Research Scientist) at Boston University have been developing a bihormonal bionic pancreas to help patients with type 1 diabetes (and insulin-dependent type 2 diabetes) control their blood glucose levels. The system integrates three components: a continuous glucose monitor, external infusion pumps to deliver insulin (a blood-sugar lowering hormone) and glucagon (a blood-sugar raising hormone) through the skin, and an adaptive mathematical algorithm that they developed to automatically administer insulin and glucagon doses to regulate the patient’s glucose levels. The group has recently developed a portable prototype of the bionic pancreas, controlled by an iPhone (see diagram below).
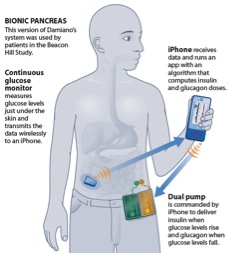
The bihormonal bionic pancreas has already undergone four years of feasibility clinical testing at MGH under the clinical guidance of Dr. Steven Russell, an endocrinologist. The group recently completed the first round of their first outpatient transitional study, called “Beacon Hill Study”. The study aims to test the iPhone-driven bionic pancreas in 20 adults in 5-day long experiments, where subjects are allowed free access to 3 square-miles in downtown Boston around MGH between 7 AM and 11 PM, and sleep in a hotel at night. Volunteers are free to engage in activities of their choice (eat and exercise freely) and are accompanied by a nurse to check their blood glucose every two hours. Volunteers spend another 5 days where they control their glucose.
The research group is about to start another transitional trial this summer at two diabetes camps in central Massachusetts (Camp Joslin and Clara Barton Camp), which will include 32 children, 16 boys and 16 girls. In each camp, 8 of the 16 volunteers will first wear the bionic pancreas continuously for 5 days, and then follow with another 5 days of usual care where they control their glucose, while the other 8 volunteers will simultaneously do the same but in reverse order. For comparison, volunteers will wear the same continuous glucose monitoring system when on the bionic pancreas as well as during usual care. They will participate in normal camp physical activities and be served typical camp meals during the entire study.
The group plans to follow the camp study with their final transitional study, in which they will enroll hospital staff with type 1 diabetes at four medical facilities, before they move on to their pivotal study in 2015, and submit for FDA for approval of the bionic pancreas in 2016. The group estimates a commercial launch in 2017.
For more information on Boston University’s bihormonal bionic pancreas, you can visit: http://bionicpancreas.org/

3 Comments
Andrew Morrow posted on July 9, 2013 at 9:47 am
Seems like the timeline is slightly behind the Artificial Pancreas Project (APP) that’s had a recent clinical trial in Virginia. How are these projects the same, or different? Has someone published any comparisons? Do you anticipate overlap in the COTS technologies (i.e. you are on iPhone, they are on Android, I believe)?
Mike Errigo posted on July 10, 2013 at 2:45 pm
This is very exciting news. @ Andrew Morrow: I believe the Artificial Pancreas Project does not include the use of Glucagon at this time. For lows, or predicted lows, insulin is suspended. Also, there are probably differences in the control algorithm as well as how the algorithm controls BG levels. For example, some correct to a target BG level (say 100 mg/dl) while others correct to a range (say 80-180 mg/dl). As for the specifics of these two systems, I am not sure.
Rachel W posted on June 4, 2015 at 3:34 pm
This is an article from two years ago, so I was curious as to the progress on this device. I am doing research on safety factors for type 1 diabetes devices and patient environments. Any help you could provide would be appreciated. Thanks!