|
December 2014
My research has been profiled in the newest issue of Bostonia magazine. Check it out here.
.
November 2014
“Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles” has been accepted at Global Change Biology. Co-authors include Adrien Finzi, Edward Brzostek, Bridget Darby, Kim Spiller, Mark Kramer and Rich Phillips. My other paper “Are above and belowground phenology in sync?” can be accessed at New Phytologist or downloaded here. Here is a sneak peek looking at patterns of root and shoot seasonality across biomes:
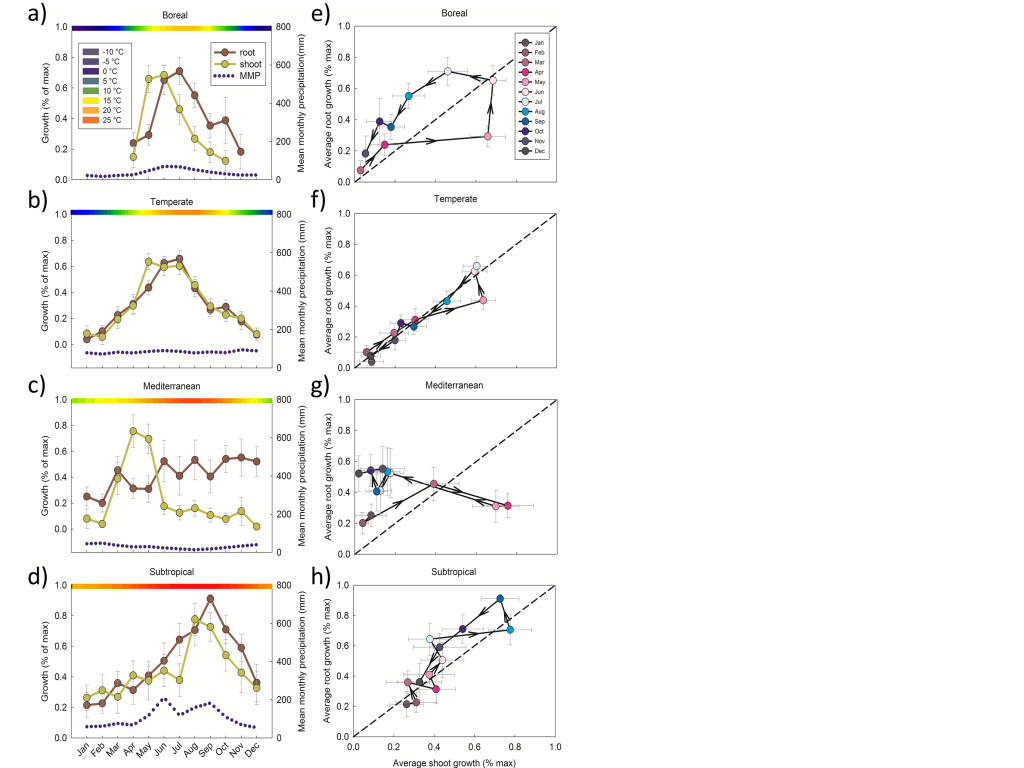 Panels a-h plot the proportion of maximum monthly root and shoot growth in four biomes. The blue dotted line is mean monthly precipitation (mm, right side y-axis) and the color bar across the top is temperature (°C). Panels e-h plot the proportion of maximum monthly root vs. shoot growth. In these panels, black lines join consecutive months and the direction of the arrowheads indicates time from Jan to Dec. September 2014
“Are above and belowground phenology in sync?” was accepted at New Phytologist. This paper uses meta-analysis to quantify the relationship between root and shoot phenology across four biomes. You’ll have to read it for the details, but answer to the tantalizing title question is best expressed by this amazing abstract: Probably not. Adrien Finzi is a co-author. Thanks to all who gave feedback!
.
August 2014
This month, I presented my paper “Are above and belowground phenology in sync?” at the organized session “A path forward for the improved representation of fine roots in terrestrial biosphere models”. I’m excited to submit my data to a root trait database that will be hosted by ORNL and incorporated into TRY!
.
April 2014
First root measurements this month, and great discussions at the Northeast Natural History Conference in Springfield, MA, where I was invited to give a talk on some of my root phenology work. I’m also happy to announce that I won the American Association of University Women (AAUW) Doctoral Dissertation Fellowship – which means I get financial support during the coming year of writing – as well as access to a network of powerful women.
.
March 2014
This month, I presented an updated version of my work at the annual meeting of our DOE funded “Partitioning CO2 fluxes with isotopologue measurements and modeling to understand mechanisms of forest carbon sequestration” with Scott Saleska, Eric Davidson, Kathleen Savage, Marc-Andre Giasson, Adrien Finzi, Rich Wehr, Daniel Scott and Paul Moorcroft.
I also sat in on some great talks at the Harvard Forest Ecology Symposium. .
January 2014
Happy New Year! If you happen to be at Harvard Forest on 1/14, please join me from 2-4pm for a Harvard Forest Lab Group that I’m leading in the seminar room. A summary of what I’ll be discussing can be downloaded here: HF Lab Meeting Blurb.
.
December 2013
I gave an oral presentation at the AGU Fall Meeting entitled “Root phenology at Harvard Forest and beyond” which won an Outstanding Student Paper Award. You can download the powerpoint and leave comments at my AGU OSPA page. Great to see everyone at this meeting.
.
October/November 2013
Busy tucking in my plots for the winter, gathering some last few respiration measurements before snow hits. Also, Finzi Lab now has a lab website, thanks to Mustafa Saifuddin, our newest PhD student. It’s still new, but you can find research profiles and publications for Finzi lab members there.
.
September 2013
Research continues with UROP student Aubree Woods who wants to know if root competition between dune grasses leads to overyielding. The Massachusetts Department of Conservation and Recreation is actively working on management strategies to stabilize coastal dunes during storm events. If interplanting grasses causes greater root growth, then it may be a viable management strategy. We received permission from the DCR to excavate sand cores from mixed and single species of dune grasses at Sandy Point State Reservation. In order to do this, we had to spend a beautiful day on the beach, driving past lobster shacks and sunflower fields. Poor us!
July/August 2013
This month we are measuring the in situ activity of four exoenzymes produced by microorganisms that live in the soil, 1) acid phosphatase, 2) aminopeptidase, 3) beta-glucosidase, and 4) chitinase, using a method adapted from Dong et al. (2007). Using chromatography paper soaked in substrates that are fluorescent or colorimetric conjugates (depending on the target enzyme), we can determine enzyme activity by comparing images of paper incubated in situ to standards that have been degraded with known quantities of the target enzyme. We are using these data to compare enzyme activity in white ash (Fraxinus americana), red oak (Quercus rubra) and eastern hemlock (Tsuga canadensis) stands at Harvard Forest, which have different mycorrhizal associations. We can also use images of the root distribution in each root box to determine how enzyme activity changes with proximity to roots.
June 2013
The summer is in full swing out at the forest. Arline and Johanna took a quick break from all things rhizotron to help BU UROP student Aubree Woods and I find some plots for a root competition study. The sites we chose can be accessed in two ways: 1) a 30 minute stroll through the woods on a cleared path, 2) by clambering over the dammed up edge of a pond. Guess which one we prefer?
Here are Aubree, Arline, and Johanna (L to R), having conquered the waterway behind them.
Also, check out Arline’s blog post for the student perspective on our summer research.
May 2013
My fearless REU students are here! Arline Gould and Johanna Recalde have been working hard in difficult weather and seem excited about the projects that we’ll be working on this summer. We’ve already seen our first earthworm in our ash plots. In the tube window, it looks like quite a monstrosity.
In less professional news, I, having grown bored with houseplants, have decided to cultivate housemushrooms. My mycelial mat just started fruiting!
April 2013
I was out at Harvard Forest last week and got stuck in a traffic jam behind two moose on Pierce Road. I also managed to corner this little centipede in one of my tubes in the heated + N addition plots.
March 2013
Spring break! I was out in the peaceful snowy forest taking an early round of root photos and respiration measurements. Did a bit of snowshoeing up around my plots . It was bright and beautiful – I think I even got a bit of a tan! What more can you ask?
I’ll be showing a poster at the HF Ecology Symposium comparing the root phenology of hemlock and oak stands – and moderating a lunch for graduate students.
November 2012
We installed minirhizotron tubes into the long term soil warming + N addition plots at Harvard Forest to look at the impacts of temperature and nutrient addition on root dynamics. Here are some highlights:
 Andy and Allison positioning the soil corer. Tubes are inserted at a 45 degree angle to allow the soil to fall flush against the upper surface of the tube, and still capture root growth at depth.  Pat inserting a minirhizotron tube into the ground. The portion of the tube that remains above the soil surface is painted black to prevent light from reaching roots, then white to prevent the tube from absorbing and conducting heat into the soil. Including these plots, we have a total of 59 tubes at Harvard Forest. Special thanks to everyone who helped to install: Marc-Andre Giasson, Allison Gill, Pat Sorensen, Andy Reinmann, Xi Yang, Tim Savas, and Mel Knorr.
August/September 2012
Here are some photos of Sam working with the camera and a wonderful zoom shot of mycorrhizal hyphae colonizing a root tip. I’ve taken some of our preliminary findings to ESA in Portland, OR and will be presenting a poster at the LTER All Scientist’s Meeting in Estes Park, CO.
June 2012
Starting my first season as a mentor for the Harvard Forest REU program, working with Sam Knapp, from the University of Wisconsin. He is spending considerable time in the forest with our minirhizotron camera, and being supremely helpful during my other sampling campaigns. Recently, root exudates:
Have a look at Sam’s blog post to read about his experience firsthand.
May 2012
As the school year wraps up, so does my teaching experience with the NSF GK12 fellowship program. For lesson plans and highlights of my year spent in a 5th grade science classroom, please visit my GK12 GLACIER site.
Some pictures from the root box installation:
|