In early October, Japanese scientist, Shinya Yamanaka shared the Nobel Prize for medicine or physiology with British researcher, John Gordon for discovering that bodily cells, such as skin or hair cells can be reprogrammed into stem cells: a brilliant discovery that has the potential of embryonic-like cells that can become any kind of tissue.
When Stockholm’s Karolinska Institute announced the $1.2 million award, they stated that Yamanaka’s 2006 discovery of four transcription factors could degenerate mature cells into primitive cells, induced Pluripotent Stem (iPS) cells, which could be reprogrammed into different kinds of mature cells has “revolutionized our understanding of how cells and organisms develop.”
In the last few years, Boston University’s Center of Regenerative Medicine has built on this technology, namely by making it more efficient and expanding the applications—10 times higher than previously reported studies. Prior research studies relied on four different transcription factors in separate virus vectors to transfer genes into cell’s DNA. Gustavo Mostoslavsky’s, Boston University Assistant Professor of Medicine in the Gastroenterology Section, milestone discovery places all four transcription factors in one virus vector coined the “stem cell cassette or STEMCCA.”
“The use of a single lentiviral vector for the derivation of iPS cells will help reduce the variability in efficiency that has been observed between different laboratories, thus enabling more consistent genetic and biochemical characterizations of iPS cells and the reprogramming process,” the researchers concluded.
For more information, please click here.
 As we enter the Presidential election period, perhaps the most important election in a generation, at stake is contrasting visions of the role of government. After WWII, Vanevar Bush and others pushed for an activist role for the federal government in fostering basic research in the US. NSF and other government labs were formed then, followed by NIH, DARPA and NASA in the 1950s. By all measures the return on taxpayer investments has been phenomenal. Technological innovation has powered growth in industry and made our standard of living the envy of the world. The world also benefited from our basic research with huge improvements in better health and poverty reduction.
As we enter the Presidential election period, perhaps the most important election in a generation, at stake is contrasting visions of the role of government. After WWII, Vanevar Bush and others pushed for an activist role for the federal government in fostering basic research in the US. NSF and other government labs were formed then, followed by NIH, DARPA and NASA in the 1950s. By all measures the return on taxpayer investments has been phenomenal. Technological innovation has powered growth in industry and made our standard of living the envy of the world. The world also benefited from our basic research with huge improvements in better health and poverty reduction.
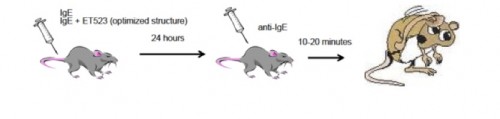
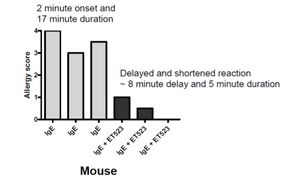
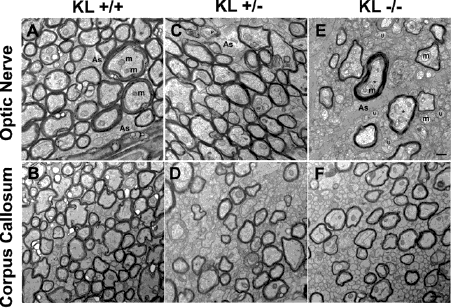 Carmela Abraham's work with the protein Klotho has provided a potential method of alleviating cognitive decline in aging organisms. In the lab, Abraham discovered a deficiency of Klotho in aging monkeys and mice, which seemed to correlate with the development of cognitive decline. Furthermore, she found that overexpression of Klotho resulted in a 30% increase in lifespan of transgenic mice. Her proposed product would utilize these findings to improve the overall health and mental well-being of any organism by elevating its levels of Klotho.
Carmela Abraham's work with the protein Klotho has provided a potential method of alleviating cognitive decline in aging organisms. In the lab, Abraham discovered a deficiency of Klotho in aging monkeys and mice, which seemed to correlate with the development of cognitive decline. Furthermore, she found that overexpression of Klotho resulted in a 30% increase in lifespan of transgenic mice. Her proposed product would utilize these findings to improve the overall health and mental well-being of any organism by elevating its levels of Klotho.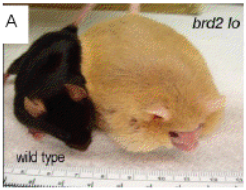 Gerald Denis has used genetic manipulation of the BET protein family to uncouple obesity from Type-2 Diabetes and inflammation. They have produced mice that are metabolically healthy despite being severely obese—by suppressing the expression of Brd2 (a member of the BET family), these mice are protected from Type-2 Diabetes. Denis proposes to use these findings to produce a few BET inhibitor compounds which could help prevent or alleviate those at risk (or suffering from) Type-2 Diabetes.
Gerald Denis has used genetic manipulation of the BET protein family to uncouple obesity from Type-2 Diabetes and inflammation. They have produced mice that are metabolically healthy despite being severely obese—by suppressing the expression of Brd2 (a member of the BET family), these mice are protected from Type-2 Diabetes. Denis proposes to use these findings to produce a few BET inhibitor compounds which could help prevent or alleviate those at risk (or suffering from) Type-2 Diabetes. As Boston University’s Director of New Ventures at the Office of Technology Development, my focus is to help build new companies based on technologies being created at the University. I was excited by the tremendous interest of faculty in participating in the creation of new ventures. I’ve found our experienced researchers are keen on finding new ways to impact society and translate their cutting-edge research into influential ventures. BU thrives on the spirit of “One BU,” where an engaged and collaborative community is also helping to create fertile ground for cultivating the next big idea.
As Boston University’s Director of New Ventures at the Office of Technology Development, my focus is to help build new companies based on technologies being created at the University. I was excited by the tremendous interest of faculty in participating in the creation of new ventures. I’ve found our experienced researchers are keen on finding new ways to impact society and translate their cutting-edge research into influential ventures. BU thrives on the spirit of “One BU,” where an engaged and collaborative community is also helping to create fertile ground for cultivating the next big idea.