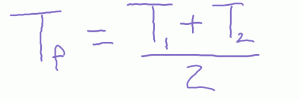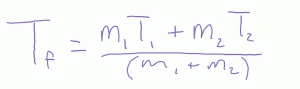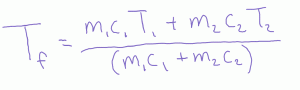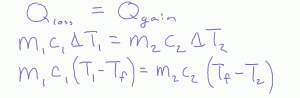So when we mix hot water with cold water there will be a transfer or thermal energy from hot to cold, or a flow of heat from hot to cold.
It takes about 4,186 Joules to heat up 1 kilogram of water by 1°C. That might not be an easy number to remember. However, it only takes 1 calorie of heat to warm up 1 gram of water by 1°C. Be careful not to confuse Calorie with a capitol C with calorie.
1 Calorie = 1 kilocalorie = 1000 calories
Keep in mind, that for most experiments we can assume (perhaps falsely) that the density of water is 1 gram/milliliter. However, if you want to be precise, you should account for this variability in experiments. Wikipedia has a nice table of densities.
When discussing heat flow, we use the equation
Q = mcΔT
where Q is the flow of heat, m is mass, c is the specific heat, and T is the temperature. As we are examining changes in temperature and not absolute temperature, it is okay to use the Celsius scale instead of Kelvin. Make sure to note if you are using Joules or calories.
You can think of specific heat as a thermal inertia. Joseph Black first noted the specific heat of substances and that adding heat would increase the temperature of an object. He contrasted this with Latent (or hidden) heat which can cause a phase change, but does not change the temperature.
You can view a table of specific heat on hyperphysics. Water actually has a very high specific heat compared to most substances. The reason for this has to do with the number of degrees of freedom of the molecules. If I add a calorie of heat to a gram of water and a calorie to a gram of aluminum, the aluminum will warm up 5 times as much! Copper 10 times as much!
The numbers worth remembering:
c(water) = 4187 J/kg/°C = 1 cal/g/°C
c(ice) = 2090 J/kg/°C = 0.5 cal/g/°C
Although these numbers themselves change slightly with temperature, we will assume they are constant.
Be cautious not to confuse specific heat with conduction. You will burn yourself easily on the metal pan because it conducts its heat to you quickly, not because it is at such a high temperature.
When heat flows into an objects you are increasing its thermal energy. In the case of a solid it is vibrating in three dimensions. In a liquid, you might also have the addition of rotation and translation of the molecules.
There is an interesting relationship for the specific heat of metals called the Law of Dulong and Petit. Although it seems that the specific heat varies greatly for different metals this is because of the mass of the atoms. The specific heat per mole of molecules does not vary much.
A mole is a way my chemistry friends can avoid counting over 10. One moles is 6.02 x 10^23 molecules, which is also known as Avocado’s number.
There are a lot of fun experiments we could do to do work on an object and see the resulting change in temperature.
We could stretch and deform a rubber band.
We could measure the change in temperature in a can full of sand by shaking if for a few minutes. Or even just drop if from a large height. Potential energy becomes heat!
We could hit a nail with a hammer into hard wood. Feel it.
When we ran current through the resistors, you could feel the heat. We could measure that temperature. Or a nichrome wire would really heat up!
We also have a bunch of submersible light-bulbs by PASCO that we can measure the power going into the bulb and the heat coming out! One should read through the user manual first.
So what are some cool mixing things you could do.
What happens when you mix equal volumes of hot water and cold water? We just have the average temperature.
What happens if you mix unequal volumes of hot water and cold water? Here we have a weighted average.
What happens if you mix water with something else? How about cold water and hot steel nails? Or hot water with cold alcohol? Then we need to account for this thermal inertia so we have an weighted weighted average (weighted by specific heat).
Beyond saying this formula is a weighted average, what is the physics behind it? When we mix hot and cold fluids, one gains heat and the other loses heat. Assuming no losses and these heats are equal.
Now we distribute
Next we move the initial temperatures to one side, and the final temperature terms to the right.
Finally, we factor
And in our last step we wind up with the equilibrium formula for mixing.
What if you mix warm water with an ice cube?
Then we have to account for the latent heat.
Instrumentation:
Vernier Probes: The stainless steel probe contains a thermistor at the end.

I just picked up some new toys from National Instruments, their MyTemp. You can learn a bit more here. These are thermistors which are different than the Vernier probes. They don’t have the long rod so you can put them in various places.